Pianos and Periodic Tables:
How to Best Learn Chemistry at Home
by: Dr. Colleen Kelley
Do you have a memory of the first time you sat down at a keyboard and tried to play music? Do you remember the cacophony of sounds coming from the keyboard? Was it music or noise? Most likely it was noise. What did you want to know next? You probably wanted to know how people could press the keys so that songs were heard. This, of course, required instructions otherwise known as the musical notes found in a composed piece of music. The transition between randomly hitting keys on a keyboard (or plucking strings on a guitar) and playing music comes from learning how to read and interpret musical symbols. This process takes many years and requires learning, practicing, and becoming ‘fluent’ in the language of music. The same is true for chemistry.
Mixing chemicals from a purchased chemistry kit so that your child can be exposed to chemistry is much like putting them in front of a piano and pounding the keys. You child will have fun mixing chemicals to cause explosions, change colors, create smells, or make a goo. Is this chemistry or a mess? Most likely it is just a mess. Chemistry, like music, requires an understanding of the visible, tactile phenomena. Like music, the transition between playing with a chemistry kit and understanding chemistry comes from learning how to read and interpret chemical symbols. This process also takes many years and requires learning, practicing, and becoming ‘fluent’ in the language of chemistry.
We have known for decades that the best time for kids to learn music is between the ages of 4 –
10 when their brains have the plasticity to interpret musical symbols to develop fluency. We also
recognize that kids need to continue to learn, practice, and compose music to maintain or
increase that fluency. No students go to college to major in music without ever having played an
instrument! Can you imagine majoring in music and never having read music? Well, this is exactly what is happening in chemistry.
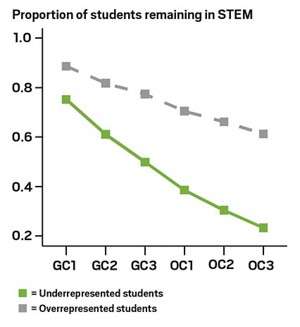
Many students, especially those in underrepresented groups, go to college to major in science without an understanding of chemistry. We expect them to become ‘fluent’ in a single semester. It’s no wonder that the failure rate for underrepresented students is 80% after two years of college chemistry. They have not had the years of practice required to excel in this course. And the stakes are high – chemistry is a pre-requisite for all science and engineering majors. You can’t be a doctor, nurse, scientist, or engineer unless you pass chemistry. Failing chemistry costs these students millions of dollars in income over a lifetime. And worse than that….it squashes their dreams and confidence. Fundamentally, this is an issue of civil rights.

Science educators have been working for years to solve this problem. Most of the energy is put towards trying to ‘save’ the failing college students. This has not been successful because it’s already too late. There are no shortcuts to symbolic learning. One tutor or a few YouTube videos does not solve this problem. So, how can we solve this problem? We need a revolution in science education so that we think of learning chemistry more like learning music than math.
This revolution involves the introduction of chemical symbols close to the same time we introduce musical symbols – between the ages of 8 and 12. How can we do this? I’ve created a series of ten chemistry comic books that scaffold the concepts found in a college-level chemistry course. In these comic books molecules are quirky characters
with superhero-like powers. Elements are members of a heavy metal band. Chemical reactions play out in suspenseful (and hilarious) story plots rather than being a bunch of undecipherable symbols stagnant
on a page.
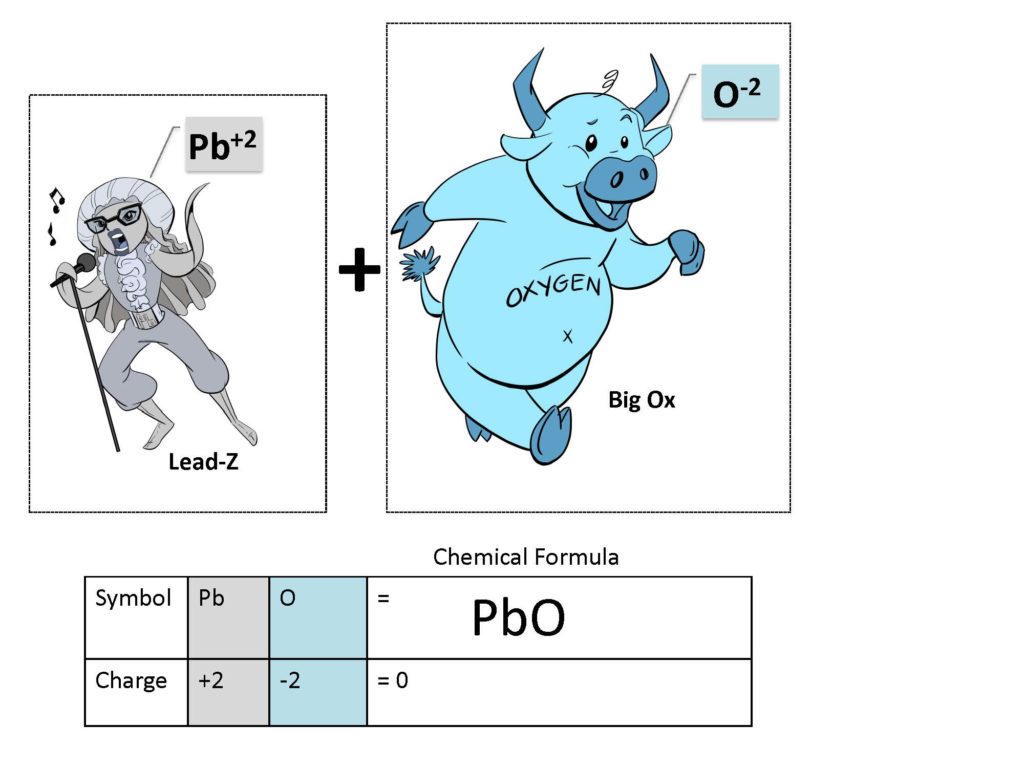
How does this work? One of the most problematic skills for new students to develop is that of balancing chemical equations. The concept is simple: you need to have the same number of the same kinds of atoms on both sides of an equation. It becomes balanced like a seesaw. For example, if there are 2 atoms of sodium (Na) on one side of the equation, there needs to be 2 atoms of sodium (Na) on the other side of the equation. This is due to the Law of Mass Balance which basically states that matter cannot be created or destroyed. I use this story to describe the Law of Mass Balance. Imagine making chocolate chip cookies. You put in 100 chocolate chips in the batter. Assuming no one eats the dough, or no dough is otherwise lost, the entire batch of baked cookies also has 100 chocolate chips. Before = After. There is not a gnome in your oven stealing chocolate chips out of the dough nor is there a gnome putting more chips into the cookies. All students comprehend before equals after. Then why is it so hard for students to balance chemical equations? It took me a long time (OK, 25 years of teaching to be exact! Ugh!) to figure out that the difficulty came from the fact that students could not ‘see’ what they were balancing. Students were having trouble differentiating between the sea of chemical symbols in the equation and therefore couldn’t discern which was which to balance! Let’s look at this example.
This approach works with kids ages 8 – 12! In fact, the 8 – 12 years old kids who have already learned from these comic books describe the experience as “fun, really fun”.
The first comic books were piloted with a group of students ages 8, 9, and 10. I instructed parents to be ‘hands off’ and allow their children to read the mysteries much like they would read any comic book. I didn’t want the comic books to be thought of as homework or something that would be graded. Instead, I wanted to see what each child could learn just by enjoying the mystery and the characters within the stories. What I found amazed me! I had a book-club by Zoom with each student just to chat about each mystery. They would tell me how to calculate the number of protons, neutrons, and electrons in an atom, how to write a formula for a complex ionic compound, and how the structure of the atom is ‘cloud-like’.
The comic book series is titled The M.C. Detective Agency: Chemical Solutions Required and envelops a cleverly concealed chemistry curriculum within the stories of twins, Poppi and Ray, who solve mysteries using chemistry. These sibling sleuths have many adventures, including traveling back in time to rescue the Radium Girls, attending a modern-day rock concert to save a vanishing Van Gogh, and swimming in a bottle of Chanel No. 5 to find the hiding aldehydes. The readers eventually discover that M.C. is Marie Curie, hence the names Poppi (Polonium), Ray (Radium) and Granny Eve (Marie’s
Curie’s youngest daughter).


The comic book series creates a platform for understanding chemistry concepts in a fun, enjoyable, robust, and memorable way. Readers of this series will be able to thrive in chemistry courses in high school and college. Most importantly, they will be able to understand and write a symphony of chemical reactions to explain the process of not only making goo but the myriad scientific concepts that our world depends upon. This series will create a generation of literate and fluent science speakers…. something the world needs.
Dr. Colleen Kelley
Think chemistry is boring? Think again! A class with Chemistry and Biochemistry at The University of Arizona instructor, Colleen Kelley, is filled with colorful characters and exciting storylines that translate complex chemistry into comic books Her comics and unique imagination have turned the periodic table into a playground of chemical adventure, and have allowed elementary school students to master concepts often taught at the college level.